A lung-inspired design turns water into fuel

The act of inhaling and exhaling is so automatic for most organisms that it could be mistaken as simple, but the mammalian breathing process is actually one of the most sophisticated systems for two-way gas exchange found in nature. With each breath, air moves through the tiny, passage-like bronchioles of the lungs until it reaches diminutive sacs called alveoli. From there, the gas must pass into the bloodstream without simply diffusing, which would cause harmful bubbles to form. It's the unique structure of the alveoli - including a micron-thick membrane that repels water molecules on the inside while attracting them on the outer surface -- that prevents those bubbles from forming and makes the gas exchange highly efficient. Scientists in senior author Yi Cui's lab at the Department of Materials Science and Engineering at Stanford University drew inspiration from this process in order to develop better electro catalysts: materials that increase the rate of a chemical reaction at an electrode. "Clean energy technologies have demonstrated the capability of fast gas reactant delivery to the reaction interface, but the reverse pathway - efficient gas product evolution from the catalyst/electrolyte interface - remains challenging.
The team's mechanism structurally mimics the alveolus and carries out two different processes to improve the reactions that drive sustainable technologies such as fuel cells and metal-air batteries. The first process is analogous to exhalation. The mechanism splits water to produce hydrogen gas, a clean fuel, by oxidizing water molecules in the anode of a battery while reducing them in the cathode. Oxygen gas (along with the hydrogen gas) is rapidly produced and transported through a thin, alveolus-like membrane made from polyethylene -- without the energy costs of forming bubbles.
The second process is more like inhalation and generates energy through a reaction that consumes oxygen. Oxygen gas is delivered to the catalyst at the electrode surface, so it can be used as reactant during electrochemical reactions.
Source: https://www.sciencedaily.com/releases/2018/12/181220111754.htm
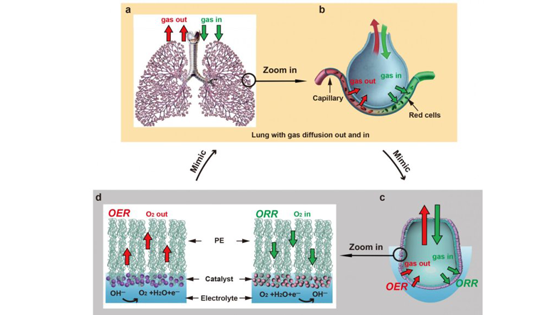
Image Source : https://www.sciencedaily.com/releases/2018/12/181220111754.htm

